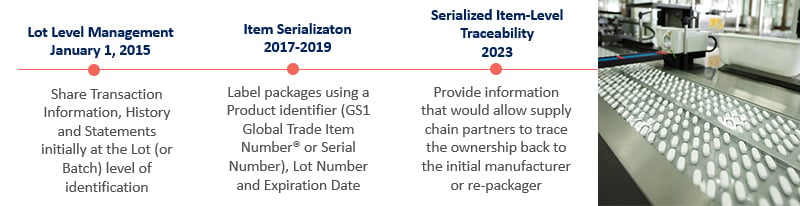
We understand that becoming compliant with new DSCSA regulations and GS1 standard is mission critical for your business. As of 2017, all pharmaceutical manufacturers need to label all packages using a product identifier (serial number), lot number and expiration number - and track them to the lowest "sellable" unit of measure.
Ready or not, this mandate is coming.
Our new Track and Trace module offers the combination of our Oracle Validated Integration to JD Edwards with the benefit of regulatory compliance for serialization. With RF-SMART, JD Edwards is updated direcly, in real-time, with material movement and inventory changes. This provides you greater visibility of invetory and improved capabilites to track and measure.
© 2026 RF-SMART. All rights reserved. Privacy Policy